This is the first week of creating content for the blog as we figure out the direction that we want to take. This year, the group has grown from five members to twelve members, and so in order to utilize our manpower effectively, we’ve decided to split our team into three subgroups and work on similar objectives and compare our results. For next week, the groups will finish up CAD designs of a water electrolysis device.
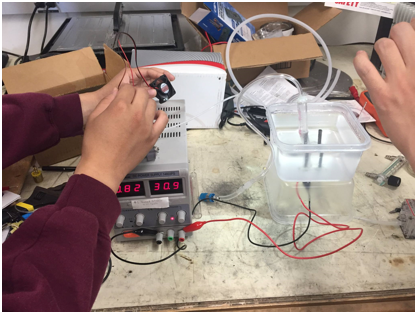
In these past two weeks, we started off with the basics by learning the fundamentals and theory to help us better understand fuel cells. We started off by getting to know and learn more about its concepts and practice through articles shared within the group to facilitate thinking and discussion. For further exposure to this topic, we conducted an experiment on water electrolysis which is one of the main process in producing and providing sufficient electricity for a fuel cell.
In the electrolysis lab, we placed two conducting rods in water and connected one to positive and the other negative through a power supply. As we run the power supply, we can see some formation of bubbles that indicate the start of water electrolysis. In this chemical reaction, water is being separated into oxygen gas and hydrogen ions at the anode(positive). The oxygen stays while the positively charged hydrogen ions want to move towards the cathode(negative). As the hydrogen ions combine with electrons provided from the power source, it produces hydrogen gas with stored energy inside the chemical bonds. We also added salt to increase the conductivity of the solution that would allow more current flow to produce more gas. The gas was then transferred through a tube to the fuel cell to power a small fan, but was unsuccessful in getting it to work. After looking over the system for the any errors or mistakes, there was none and we concluded that the fuel cell was defective and needed to be replaced.
Overall, we got the gist of what and how a water electrolysis works and its application towards a fuel cell despite the failure at the end of the experiment. Therefore, we are ready to start designing a fuel cell on CAD and work on multiple simulations to try to achieve efficiency as high as possible while taking into account of our resources and limitations. In the end, we will choose two out of the three to test and the test parameters of the upcoming experiment are: water temperature from cold to hot, distance between the catalyst, and lastly will be the pressure of hydrogen. We will use all of this to test the efficiency of production of hydrogen as fuel for the fuel cell.